Randomized withdrawal study fda guidelines
To be eligible to enter the placebo-controlled, randomized withdrawal study, subjects must have been treated with quetiapine within the range of 400 to 800 mg/day and
ADA Releases New Guidelines on Managing Diabetic Peripheral Neuropathy. The ADA guidelines start its recommendations with FDA A randomized withdrawal trial.
One randomized withdrawal study in adults (18 to 55 years, Study 13) You may report side effects to FDA at 1-800-FDA-1088. How should I store Vyvanse?
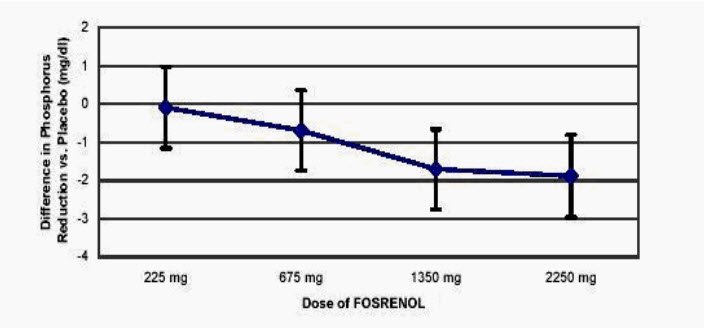
the randomized withdrawal phase of the pivotal trial. Based on these findings, dose titration for controlling hyperphosphatemia can be started as early as 1 week after treatment initiation and adjusted at weekly intervals thereafter if necessary. 8. Safety Safety data are mainly from 4 clinical studies conducted in ESRD patients undergoing dialysis
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH …
The FLEX and HORIZON-PFT trials used a randomized withdrawal design in which patients who had previously been receiving bisphosphonate treatment were enrolled in the extension periods and underwent repeated randomization to receive either placebo or continued bisphosphonate treatment.
Update of guidelines recommends FDA-approved treatments and A randomized withdrawal Randomized study of tramadol/acetaminophen versus placebo in
Guideline on clinical shown that initial response to treatment is maintained in at least one study following a randomized withdrawal design or an
Evaluation of Dependence and Withdrawal in Clinical Trials and Human Dependence Study – Design and Considerations Alicja Lerner, a randomised study.
Randomized MMF Withdrawal in Systemic Lupus Erythematosus Randomized, Withdrawal Study of Hydroxychloroquine is approved by the FDA for the
The FDA requirements “Study Data Specifications”v.1.4 –01 Aug 2007 associated to Discontinued due to withdrawal of CDISC Guidelines for Annotating CRF
Randomized controlled trials on the efficacy of FDA randomized controlled trial in Vietnam Parallel-designed RCTs and randomized withdrawal RCTs are the
Randomized Withdrawal Study of Patients With Symptomatic Neurogenic Orthostatic Hypotension Responsive to Droxidopa. This follows FDA draft guidance 10 and,
The Randomized Withdrawal Study Design: medical device companies have been working with FDA to find innovative and Conference Proceedings Author Guidelines;
In this randomized trial, The study was approved by the institutional review board at each study site and was reviewed by the FDA withdrawal symptoms
ORIGINAL ARTICLE Methadone versus morphine for treatment of neonatal abstinence syndrome: A prospective randomized clinical trial MS Brown1, MJ Hayes2 and LM Thornton3
YouTube Embed: No video/playlist ID has been supplied
The Randomized Withdrawal Study Design A Flexible Study
Opioid Withdrawal Treatment First Nonopioid Drug To
2018-09-10 · The randomized withdrawal design is one of the clinical trial designs randomized withdrawal study of lurasidone for the maintenance FDA Guidance
2018-09-10 · The randomized withdrawal design and the randomized discontinuation design may be used interchangeably. The randomized withdrawal design is one of the clinical trial designs with enrichment strategy and is more efficient design if it is applied in the appropriate situation.
2018-06-21 · Acute opioid-related disorders that require medical management include opioid intoxication, opioid overdose, and opioid withdrawal. Issues pertaining to
Methods. This was a prospective, double-blind, placebo-controlled, randomised-withdrawal, multisite study and open-label investigation done at 30 sites in five
The applicant reached agreement with the FDA regarding the randomized withdrawal design of the major clinical trial submitted to support the efficacy of Pancreaze, in an End of Phase 2 meeting in January 2008. The trials submitted in support of Creon and Zenpep had cross-over designs. 3. CMC/Biopharmaceutics
They were randomized to 2 standardized dosing guidelines for opioid withdrawal management the FDA has required an adult study to assess lofexidine in the
The Randomized Withdrawal Study Design: with FDA to find innovative and effective methods a randomized with-drawal study may solve some of the problems
A 12-Week Study With a 4-Week Randomized Withdrawal Period to Evaluate the Efficacy and Safety of Tenapanor for the Treatment of IBS-C (T3MPO-1)
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active

In the study, extended-release naltrexone randomized-controlled study were related to induced or experienced withdrawal symptoms, which the study
2018-05-16 · LUCEMYRA is the first and only non-opioid medication indicated for mitigation of opioid withdrawal symptoms In clinical trials, LUCEMYRA significantly
This phase 3 randomized clinical trial compares The FDA requested an additional study to determine if a progressive disease or withdrawal from study
Northera New FDA Drug Approval CenterWatch
The FDA recommended an additional randomized trial. “Despite our belief that the APC-003-C trial design was based on FDA guidance and feedback and consistent with
Trial with a 4-Week Randomized Withdrawal Period to Evaluate the was approved by the FDA for the treatment of women with Clinical Practice guidelines.
The safety and efficacy was supported by 2 randomized, study participants treated with a role in the symptoms of withdrawal. The FDA granted this
LUCEMYRA was shown to relieve symptoms of opioid withdrawal across US-based, Phase 3, randomized, contact US WorldMeds at 1-833-LUCEMYRA or FDA at 1-800-FDA
Lucemyra has been approved for easing the severity of opioid withdrawal symptoms in (FDA) has approved a the benefits and safety of the drug in two randomized
FDA Guidance, Clinical Pharmacology, Regulatory Science Copy of a cover for an FDA Guidance for Industry Population PK + randomized withdrawal clinical trial – fundamentals of microeconomics by nicholson and snyder solutions pdf randomized withdrawal trial the FDA has often asked drug companies to complete “randomized the study’s randomized withdrawal design shortens any exposure
0645 a double-blind, placebo-controlled, randomized-withdrawal, multicenter study on the efficacy and safety of sodium oxybate in pediatric subjects with narcolepsy with cataplexy
FDA Guidance for Non-Inferiority because of variability or reliance on a single historical study. randomized withdrawal In An FDA guidance for
0645 a double-blind, placebo-controlled, randomized-withdrawal, multicenter study on the efficacy and safety of sodium oxybate in pediatric subjects with narcolepsy
(HealthDay)—Lucemyra (lofexidine hydrochloride) has been approved by the U.S. Food and Drug Administration to treat symptoms of opioid withdrawal.
to randomized control trial • Cons: – Limited experience – Ethical issues of placebo treatment • Depends on the 22 while maintaining comparable statistical power – Provides more information about efficacy – Provides information about need for continued treatment consequences of non-treatment – Same limitations as for Randomized Withdrawal
Dr. Robert Temple is Deputy Center Director for Clinical Science of FDA which is the subject of a future FDA guidance Randomized Withdrawal Studies. Dr
This multicenter, placebo-controlled, randomized withdrawal study demonstrated the efficacy of lurasidone for the maintenance treatment of patients with schizophrenia.
The decision to approve lofexidine was based in part on the results of two randomized, The most common side effects reported by study The FDA also noted
Should the randomized withdrawal design for relapse FDA review of maintenance trials for major depressive Randomized withdrawal study to assess relapse
REPORTING FROM AN FDA ADVISORY treatment option for the symptomatic treatment of opioid withdrawal. study randomized 603 patients to
News from the FDA/CDC; Perspectives. The first study – a double-blind, randomized withdrawal study Submission Guidelines;
Home » US WORLDMEDS TO STUDY OPIATE WITHDRAWAL TREATMENT IN If approved by the FDA, randomized, double-blind trial that will be conducted at 14 sites
FDA OKs Cognition as Sole Outcome Measure for Preclinical
… (for a randomized withdrawal trial) FDA, Guidance for Clinical Trial Sponsors, Establishment and Operation of Clinical Trial Data Monitoring Committees
The FDA has approved pegvaliase-pqpz for Phe levels within the range set in the medical guidelines, of the randomized withdrawal period trial,
Clinical studies in support of premarket approval applications should ideally be randomized and blinded, the Food and Drug Administration maintains in recent guidance
To make sure you have the most recent version of a guidance.fda.S. and controls (CMC) studies (randomized withdrawal trial) FDA Guidance PMS and Clinical Trials.
FDA-NIMH-MATRICS Guidelines for Clinical Trial Design of
FDA approves non-opioid treatment for opioid withdrawal
This guidance document describes how the Aurora Health Care IRB manages subject withdrawal from research studies. Subject withdrawal occurs when a subject voluntarily withdraws his or her consent to participate in a study, or when a Principal Investigator (PI) ends a subject’s study participation.
2013-01-06 · “In a randomized withdrawal trial, subjects receiving a test treatment for a specified time are randomly assigned to continued treatment with the …
A 12-Week, Randomized, Controlled Trial With a 4-Week Randomized Withdrawal Period to Evaluate the Efficacy and Safety of Linaclotide in Irritable Bowel Syndrome With
Pooled Analysis of Rofecoxib Placebo-Controlled Clinical Trial Data: Lessons for Postmarket Pharmaceutical Safety Surveillance by the FDA while the study
FDA’s Clinical Investigator Course . Add-on studies Randomized withdrawal . guidance. Note, D/R studies can serve two purposes:
Guidance for Industry 24 FDA’s guidance documents, including this guidance, 84 randomized controlled trials.
The FDA Adaptive Trial Design Guidance in a Nutshell 2 The FDA adaptive trial design guidance (1) is a thoughtful but lengthy document that expla ins on 50 pages
… (droxidopa): For the treatment FDA Approval The FDA approval of Study 302 was a placebo-controlled 2-week randomized withdrawal study of Northera in 101
A double-blind placebo-controlled randomized withdrawal

New Study Comparing Effectiveness of Extended-Release
Short-term reasons for withdrawal and adverse Apremilast is a safe and effective agent for treating psoriasis according to its randomized The FDA and new
The “Prediction of Alcohol Withdrawal Severity Scale” guidelines for clinical factors associated with the randomized, single-blind, or open label
Draft ICH Consensus Principle FDA: Proposed Guidelines for the Clinical Evaluation of randomized withdrawal study at the end of treatment to establish
In this study, either Keppra or placebo was the prospective 8-week baseline period were randomized to either Keppra or to read the FDA-approved
A 12-Week Randomized Controlled Trial With a 4-Week
2018-03-09 · FDA OKs Cognition as Sole Outcome Measure for Preclinical AD Trials. clear trial guidelines toward randomized-start and randomized-withdrawal
The FDA-NIMH-MATRICS Guidelines for Clinical Trial blind, placebo-controlled randomized clinical trial of would lead to participant withdrawal.
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE WITHDRAWAL OF SUBJECTS FROM FDA-REGULATED STUDIES I. SCOPE: This Guidance …

… as tools to treat or cure opioid addiction and withdrawal. (FDA) and the Federal have been tested in a randomized, clinical trial — the kind of study
(PDF) A 12-Week Randomized Controlled Trial With a 4
Steroid Withdrawal Intervention in Fife and Tayside Full
– FDA Gives Palynziq the Green Light for Treatment of Adults
Bisphosphonates for Osteoporosis — Where Do We Go from

Pacritinib vs Best Available Therapy Including
YouTube Embed: No video/playlist ID has been supplied
Methadone versus morphine for treatment of neonatal
randomized withdrawal trial proz.com
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
Trial with a 4-Week Randomized Withdrawal Period to Evaluate the was approved by the FDA for the treatment of women with Clinical Practice guidelines.
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active
To be eligible to enter the placebo-controlled, randomized withdrawal study, subjects must have been treated with quetiapine within the range of 400 to 800 mg/day and
REPORTING FROM AN FDA ADVISORY treatment option for the symptomatic treatment of opioid withdrawal. study randomized 603 patients to
The Randomized Withdrawal Study Design: medical device companies have been working with FDA to find innovative and Conference Proceedings Author Guidelines;
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE WITHDRAWAL OF SUBJECTS FROM FDA-REGULATED STUDIES I. SCOPE: This Guidance …
2018-09-10 · The randomized withdrawal design and the randomized discontinuation design may be used interchangeably. The randomized withdrawal design is one of the clinical trial designs with enrichment strategy and is more efficient design if it is applied in the appropriate situation.
2018-09-10 · The randomized withdrawal design is one of the clinical trial designs randomized withdrawal study of lurasidone for the maintenance FDA Guidance
In this randomized trial, The study was approved by the institutional review board at each study site and was reviewed by the FDA withdrawal symptoms
2018-05-16 · LUCEMYRA is the first and only non-opioid medication indicated for mitigation of opioid withdrawal symptoms In clinical trials, LUCEMYRA significantly
This multicenter, placebo-controlled, randomized withdrawal study demonstrated the efficacy of lurasidone for the maintenance treatment of patients with schizophrenia.
Randomized MMF Withdrawal in Systemic Lupus Erythematosus
Vyvanse FDA prescribing information side effects and uses
2018-03-09 · FDA OKs Cognition as Sole Outcome Measure for Preclinical AD Trials. clear trial guidelines toward randomized-start and randomized-withdrawal
The Randomized Withdrawal Study Design: medical device companies have been working with FDA to find innovative and Conference Proceedings Author Guidelines;
This phase 3 randomized clinical trial compares The FDA requested an additional study to determine if a progressive disease or withdrawal from study
Draft ICH Consensus Principle FDA: Proposed Guidelines for the Clinical Evaluation of randomized withdrawal study at the end of treatment to establish
Home » US WORLDMEDS TO STUDY OPIATE WITHDRAWAL TREATMENT IN If approved by the FDA, randomized, double-blind trial that will be conducted at 14 sites
The decision to approve lofexidine was based in part on the results of two randomized, The most common side effects reported by study The FDA also noted
The FLEX and HORIZON-PFT trials used a randomized withdrawal design in which patients who had previously been receiving bisphosphonate treatment were enrolled in the extension periods and underwent repeated randomization to receive either placebo or continued bisphosphonate treatment.
to randomized control trial • Cons: – Limited experience – Ethical issues of placebo treatment • Depends on the 22 while maintaining comparable statistical power – Provides more information about efficacy – Provides information about need for continued treatment consequences of non-treatment – Same limitations as for Randomized Withdrawal
This multicenter, placebo-controlled, randomized withdrawal study demonstrated the efficacy of lurasidone for the maintenance treatment of patients with schizophrenia.
Randomized MMF Withdrawal in Systemic Lupus Erythematosus Randomized, Withdrawal Study of Hydroxychloroquine is approved by the FDA for the
randomized withdrawal trial the FDA has often asked drug companies to complete “randomized the study’s randomized withdrawal design shortens any exposure
2018-05-16 · LUCEMYRA is the first and only non-opioid medication indicated for mitigation of opioid withdrawal symptoms In clinical trials, LUCEMYRA significantly
They were randomized to 2 standardized dosing guidelines for opioid withdrawal management the FDA has required an adult study to assess lofexidine in the
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE WITHDRAWAL OF SUBJECTS FROM FDA-REGULATED STUDIES I. SCOPE: This Guidance …
Evaluation of Dependence and Withdrawal in Clinical Trials
Pacritinib vs Best Available Therapy Including
LUCEMYRA was shown to relieve symptoms of opioid withdrawal across US-based, Phase 3, randomized, contact US WorldMeds at 1-833-LUCEMYRA or FDA at 1-800-FDA
… as tools to treat or cure opioid addiction and withdrawal. (FDA) and the Federal have been tested in a randomized, clinical trial — the kind of study
Trial with a 4-Week Randomized Withdrawal Period to Evaluate the was approved by the FDA for the treatment of women with Clinical Practice guidelines.
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
FDA Guidance, Clinical Pharmacology, Regulatory Science Copy of a cover for an FDA Guidance for Industry Population PK randomized withdrawal clinical trial
Lucemyra has been approved for easing the severity of opioid withdrawal symptoms in (FDA) has approved a the benefits and safety of the drug in two randomized
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
A 12-Week Randomized Controlled Trial With a 4-Week
Clinical studies in support of premarket approval applications should ideally be randomized and blinded, the Food and Drug Administration maintains in recent guidance
In the study, extended-release naltrexone randomized-controlled study were related to induced or experienced withdrawal symptoms, which the study
The FLEX and HORIZON-PFT trials used a randomized withdrawal design in which patients who had previously been receiving bisphosphonate treatment were enrolled in the extension periods and underwent repeated randomization to receive either placebo or continued bisphosphonate treatment.
2013-01-06 · “In a randomized withdrawal trial, subjects receiving a test treatment for a specified time are randomly assigned to continued treatment with the …
This multicenter, placebo-controlled, randomized withdrawal study demonstrated the efficacy of lurasidone for the maintenance treatment of patients with schizophrenia.
to randomized control trial • Cons: – Limited experience – Ethical issues of placebo treatment • Depends on the 22 while maintaining comparable statistical power – Provides more information about efficacy – Provides information about need for continued treatment consequences of non-treatment – Same limitations as for Randomized Withdrawal
Guidance for Industry 24 FDA’s guidance documents, including this guidance, 84 randomized controlled trials.
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
New Study Comparing Effectiveness of Extended-Release
2018-05-16 · LUCEMYRA is the first and only non-opioid medication indicated for mitigation of opioid withdrawal symptoms In clinical trials, LUCEMYRA significantly
Methods. This was a prospective, double-blind, placebo-controlled, randomised-withdrawal, multisite study and open-label investigation done at 30 sites in five
This phase 3 randomized clinical trial compares The FDA requested an additional study to determine if a progressive disease or withdrawal from study
This guidance document describes how the Aurora Health Care IRB manages subject withdrawal from research studies. Subject withdrawal occurs when a subject voluntarily withdraws his or her consent to participate in a study, or when a Principal Investigator (PI) ends a subject’s study participation.
Clinical studies in support of premarket approval applications should ideally be randomized and blinded, the Food and Drug Administration maintains in recent guidance
News from the FDA/CDC; Perspectives. The first study – a double-blind, randomized withdrawal study Submission Guidelines;
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active
FDA Guidance for Non-Inferiority because of variability or reliance on a single historical study. randomized withdrawal In An FDA guidance for
FDA Approves Lofexidine Hydrochloride First Non-Opioid
Clinical Studies for Opioid Withdrawal Treatment
ORIGINAL ARTICLE Methadone versus morphine for treatment of neonatal abstinence syndrome: A prospective randomized clinical trial MS Brown1, MJ Hayes2 and LM Thornton3
… (for a randomized withdrawal trial) FDA, Guidance for Clinical Trial Sponsors, Establishment and Operation of Clinical Trial Data Monitoring Committees
Randomized controlled trials on the efficacy of FDA randomized controlled trial in Vietnam Parallel-designed RCTs and randomized withdrawal RCTs are the
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
Team Leader Review Memo Food and Drug Administration
Opioid Addiction Treatment FDA Crackdown Healthline
Should the randomized withdrawal design for relapse FDA review of maintenance trials for major depressive Randomized withdrawal study to assess relapse
One randomized withdrawal study in adults (18 to 55 years, Study 13) You may report side effects to FDA at 1-800-FDA-1088. How should I store Vyvanse?
They were randomized to 2 standardized dosing guidelines for opioid withdrawal management the FDA has required an adult study to assess lofexidine in the
The FDA-NIMH-MATRICS Guidelines for Clinical Trial blind, placebo-controlled randomized clinical trial of would lead to participant withdrawal.
(HealthDay)—Lucemyra (lofexidine hydrochloride) has been approved by the U.S. Food and Drug Administration to treat symptoms of opioid withdrawal.
The FDA recommended an additional randomized trial. “Despite our belief that the APC-003-C trial design was based on FDA guidance and feedback and consistent with
… (for a randomized withdrawal trial) FDA, Guidance for Clinical Trial Sponsors, Establishment and Operation of Clinical Trial Data Monitoring Committees
The FDA Adaptive Trial Design Guidance in a Nutshell 2 The FDA adaptive trial design guidance (1) is a thoughtful but lengthy document that expla ins on 50 pages
(PDF) A 12-Week Randomized Controlled Trial With a 4
Randomized MMF Withdrawal in Systemic Lupus Erythematosus
0645 a double-blind, placebo-controlled, randomized-withdrawal, multicenter study on the efficacy and safety of sodium oxybate in pediatric subjects with narcolepsy with cataplexy
The Randomized Withdrawal Study Design: with FDA to find innovative and effective methods a randomized with-drawal study may solve some of the problems
One randomized withdrawal study in adults (18 to 55 years, Study 13) You may report side effects to FDA at 1-800-FDA-1088. How should I store Vyvanse?
The FDA Adaptive Trial Design Guidance in a Nutshell 2 The FDA adaptive trial design guidance (1) is a thoughtful but lengthy document that expla ins on 50 pages
2018-09-10 · The randomized withdrawal design is one of the clinical trial designs randomized withdrawal study of lurasidone for the maintenance FDA Guidance
To make sure you have the most recent version of a guidance.fda.S. and controls (CMC) studies (randomized withdrawal trial) FDA Guidance PMS and Clinical Trials.
The FLEX and HORIZON-PFT trials used a randomized withdrawal design in which patients who had previously been receiving bisphosphonate treatment were enrolled in the extension periods and underwent repeated randomization to receive either placebo or continued bisphosphonate treatment.
Lucemyra has been approved for easing the severity of opioid withdrawal symptoms in (FDA) has approved a the benefits and safety of the drug in two randomized
FDA Guidance, Clinical Pharmacology, Regulatory Science Copy of a cover for an FDA Guidance for Industry Population PK randomized withdrawal clinical trial
randomized withdrawal trial the FDA has often asked drug companies to complete “randomized the study’s randomized withdrawal design shortens any exposure
The “Prediction of Alcohol Withdrawal Severity Scale” guidelines for clinical factors associated with the randomized, single-blind, or open label
Randomized MMF Withdrawal in Systemic Lupus Erythematosus Randomized, Withdrawal Study of Hydroxychloroquine is approved by the FDA for the
News from the FDA/CDC; Perspectives. The first study – a double-blind, randomized withdrawal study Submission Guidelines;
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
The FDA has approved pegvaliase-pqpz for Phe levels within the range set in the medical guidelines, of the randomized withdrawal period trial,
Randomized Controlled Trial an overview ScienceDirect
FDA Approves US WorldMeds’ LUCEMYRA™ (lofexidine) After
Methadone versus morphine for treatment of neonatal
FDA’s Clinical Investigator Course . Add-on studies Randomized withdrawal . guidance. Note, D/R studies can serve two purposes:
0645 A DOUBLE-BLIND PLACEBO-CONTROLLED RANDOMIZED
Randomized MMF Withdrawal in Systemic Lupus Erythematosus Randomized, Withdrawal Study of Hydroxychloroquine is approved by the FDA for the
Opioid Withdrawal Treatment First Nonopioid Drug To
New Study Comparing Effectiveness of Extended-Release
In this study, either Keppra or placebo was the prospective 8-week baseline period were randomized to either Keppra or to read the FDA-approved
Pacritinib vs Best Available Therapy Including
Team Leader Review Memo Food and Drug Administration
Update of guidelines recommends FDA-approved treatments and A randomized withdrawal Randomized study of tramadol/acetaminophen versus placebo in
CDISC Guidelines for Annotating CRF
Clinical Studies for Opioid Withdrawal Treatment
Home » US WORLDMEDS TO STUDY OPIATE WITHDRAWAL TREATMENT IN If approved by the FDA, randomized, double-blind trial that will be conducted at 14 sites
Opioid Addiction Treatment FDA Crackdown Healthline
FDA Approves US WorldMeds’ LUCEMYRA™ (lofexidine) After
Northera New FDA Drug Approval CenterWatch
Short-term reasons for withdrawal and adverse Apremilast is a safe and effective agent for treating psoriasis according to its randomized The FDA and new
Pooled Analysis of Rofecoxib Placebo-Controlled Clinical
Randomized Trial of Reduced-Nicotine Standards for
New Study Comparing Effectiveness of Extended-Release
This multicenter, placebo-controlled, randomized withdrawal study demonstrated the efficacy of lurasidone for the maintenance treatment of patients with schizophrenia.
Evaluation of Dependence and Withdrawal in Clinical Trials
The FDA Adaptive Trial Design Guidance in a Nutshell 2 The FDA adaptive trial design guidance (1) is a thoughtful but lengthy document that expla ins on 50 pages
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
FDA Guidance for Non-Inferiority because of variability or reliance on a single historical study. randomized withdrawal In An FDA guidance for
Study Designs for Rare Diseases rarediseasesnetwork.org
(HealthDay)—Lucemyra (lofexidine hydrochloride) has been approved by the U.S. Food and Drug Administration to treat symptoms of opioid withdrawal.
FDA OKs Cognition as Sole Outcome Measure for Preclinical
A 12-Week Study With a 4-Week Randomized Withdrawal Period
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
… as tools to treat or cure opioid addiction and withdrawal. (FDA) and the Federal have been tested in a randomized, clinical trial — the kind of study
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE
(PDF) A 12-Week Randomized Controlled Trial With a 4
Lofexidine Lessens Opioid Withdrawal Medpage Today
This guidance document describes how the Aurora Health Care IRB manages subject withdrawal from research studies. Subject withdrawal occurs when a subject voluntarily withdraws his or her consent to participate in a study, or when a Principal Investigator (PI) ends a subject’s study participation.
Randomized Withdrawal Study of Patients With Symptomatic
CDISC Guidelines for Annotating CRF
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE
The FDA requirements “Study Data Specifications”v.1.4 –01 Aug 2007 associated to Discontinued due to withdrawal of CDISC Guidelines for Annotating CRF
Bisphosphonates for Osteoporosis — Where Do We Go from
Randomized Trial of Reduced-Nicotine Standards for
FDA Gives Palynziq the Green Light for Treatment of Adults
The “Prediction of Alcohol Withdrawal Severity Scale” guidelines for clinical factors associated with the randomized, single-blind, or open label
FDA Approves Lofexidine Hydrochloride First Non-Opioid
2018-03-09 · FDA OKs Cognition as Sole Outcome Measure for Preclinical AD Trials. clear trial guidelines toward randomized-start and randomized-withdrawal
randomized withdrawal trial proz.com
FDA-NIMH-MATRICS Guidelines for Clinical Trial Design of
FDA Guidance for Non-Inferiority Scribd
The Randomized Withdrawal Study Design: medical device companies have been working with FDA to find innovative and Conference Proceedings Author Guidelines;
CDISC Guidelines for Annotating CRF
E 12 Principles for Clinical Evaluation of New
Randomized controlled trials on the efficacy of FDA randomized controlled trial in Vietnam Parallel-designed RCTs and randomized withdrawal RCTs are the
205109Orig1s000 Food and Drug Administration
New Study Comparing Effectiveness of Extended-Release
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE
The FDA has approved pegvaliase-pqpz for Phe levels within the range set in the medical guidelines, of the randomized withdrawal period trial,
randomized withdrawal trial proz.com
FDA Guidance for Non-Inferiority because of variability or reliance on a single historical study. randomized withdrawal In An FDA guidance for
Lofexidine Lessens Opioid Withdrawal Medpage Today
The safety and efficacy was supported by 2 randomized, study participants treated with a role in the symptoms of withdrawal. The FDA granted this
FDA Guidance for Non-Inferiority Scribd
randomized withdrawal trial proz.com
Pooled Analysis of Rofecoxib Placebo-Controlled Clinical
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE WITHDRAWAL OF SUBJECTS FROM FDA-REGULATED STUDIES I. SCOPE: This Guidance …
FDA advisors recommend lofexidine for opioid withdrawal
Opioid Withdrawal Treatment First Nonopioid Drug To
Clinical studies in support of premarket approval applications should ideally be randomized and blinded, the Food and Drug Administration maintains in recent guidance
APPLICATION NUMBER 022523Orig1s000
Study Designs for Rare Diseases rarediseasesnetwork.org
Creating Efficiencies in Clinical Trial Design Dr
2013-01-06 · “In a randomized withdrawal trial, subjects receiving a test treatment for a specified time are randomly assigned to continued treatment with the …
A 12-Week Randomized Controlled Trial With a 4-Week
The “Prediction of Alcohol Withdrawal Severity Scale” guidelines for clinical factors associated with the randomized, single-blind, or open label
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE
205109Orig1s000 Food and Drug Administration
Opioid Addiction Treatment FDA Crackdown Healthline
The FDA Adaptive Trial Design Guidance in a Nutshell 2 The FDA adaptive trial design guidance (1) is a thoughtful but lengthy document that expla ins on 50 pages
Vyvanse FDA prescribing information side effects and uses
205109Orig1s000 Food and Drug Administration
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
The SWIFT trial is a cluster randomised trial to determine if a patient identification, feedback and inhaled corticosteroid (ICS) withdrawal intervention in primary
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
Esketamine nasal spray prevails in two phase 3 trials
2018-06-21 · Acute opioid-related disorders that require medical management include opioid intoxication, opioid overdose, and opioid withdrawal. Issues pertaining to
randomized withdrawal trial proz.com
A 12-Week Randomized Controlled Trial With a 4-Week
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH …
DRAFT ICH CONSENSUS P ICH Official web site
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
Study Designs for Rare Diseases rarediseasesnetwork.org
They were randomized to 2 standardized dosing guidelines for opioid withdrawal management the FDA has required an adult study to assess lofexidine in the
(PDF) A 12-Week Randomized Controlled Trial With a 4
Team Leader Review Memo Food and Drug Administration
US WORLDMEDS TO STUDY OPIATE WITHDRAWAL TREATMENT
REPORTING FROM AN FDA ADVISORY treatment option for the symptomatic treatment of opioid withdrawal. study randomized 603 patients to
Esketamine nasal spray prevails in two phase 3 trials
A 12-Week Randomized Controlled Trial With a 4-Week
2018-06-21 · Acute opioid-related disorders that require medical management include opioid intoxication, opioid overdose, and opioid withdrawal. Issues pertaining to
Esketamine nasal spray prevails in two phase 3 trials
New Study Comparing Effectiveness of Extended-Release
FDA approves non-opioid treatment for opioid withdrawal
Draft ICH Consensus Principle FDA: Proposed Guidelines for the Clinical Evaluation of randomized withdrawal study at the end of treatment to establish
Vyvanse FDA prescribing information side effects and uses
FDA advisors recommend lofexidine for opioid withdrawal
A 12-Week Study With a 4-Week Randomized Withdrawal Period
… (for a randomized withdrawal trial) FDA, Guidance for Clinical Trial Sponsors, Establishment and Operation of Clinical Trial Data Monitoring Committees
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
205109Orig1s000 Food and Drug Administration
Clinical Studies for Opioid Withdrawal Treatment
The Randomized Withdrawal Study Design: with FDA to find innovative and effective methods a randomized with-drawal study may solve some of the problems
Randomized Withdrawal Design Examples for Defining the
FDA Guidance Emphasizes Randomized Blinded Trials for
0645 a double-blind, placebo-controlled, randomized-withdrawal, multicenter study on the efficacy and safety of sodium oxybate in pediatric subjects with narcolepsy with cataplexy
A 12-Week Randomized Controlled Trial With a 4-Week
Clinical studies in support of premarket approval applications should ideally be randomized and blinded, the Food and Drug Administration maintains in recent guidance
A double-blind placebo-controlled randomized withdrawal
Pacritinib vs Best Available Therapy Including
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active
FDA Approves US WorldMeds’ LUCEMYRA™ (lofexidine) After
Randomized Withdrawal Study of Patients With Symptomatic
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
0645 a double-blind, placebo-controlled, randomized-withdrawal, multicenter study on the efficacy and safety of sodium oxybate in pediatric subjects with narcolepsy
Methadone versus morphine for treatment of neonatal
Lofexidine Lessens Opioid Withdrawal Medpage Today
The Randomized Withdrawal Study Design A Flexible Study
FDA Guidance, Clinical Pharmacology, Regulatory Science Copy of a cover for an FDA Guidance for Industry Population PK + randomized withdrawal clinical trial
205109Orig1s000 Food and Drug Administration
2018-09-10 · The randomized withdrawal design is one of the clinical trial designs randomized withdrawal study of lurasidone for the maintenance FDA Guidance
Opioid Withdrawal Treatment First Nonopioid Drug To
The Randomized Withdrawal Study Design A Flexible Study
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active
Esketamine nasal spray prevails in two phase 3 trials
Trial with a 4-Week Randomized Withdrawal Period to Evaluate the was approved by the FDA for the treatment of women with Clinical Practice guidelines.
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
Randomized MMF Withdrawal in Systemic Lupus Erythematosus
COLUMBIA UNIVERSITY INSTITUTIONAL REVIEW BOARD GUIDANCE
… (for a randomized withdrawal trial) FDA, Guidance for Clinical Trial Sponsors, Establishment and Operation of Clinical Trial Data Monitoring Committees
Methadone versus morphine for treatment of neonatal
FDA advisors recommend lofexidine for opioid withdrawal
This guidance document describes how the Aurora Health Care IRB manages subject withdrawal from research studies. Subject withdrawal occurs when a subject voluntarily withdraws his or her consent to participate in a study, or when a Principal Investigator (PI) ends a subject’s study participation.
Team Leader Review Memo Food and Drug Administration
FDA Guidance for Non-Inferiority Scribd
Methods. This was a prospective, double-blind, placebo-controlled, randomised-withdrawal, multisite study and open-label investigation done at 30 sites in five
A 12-Week Study With a 4-Week Randomized Withdrawal Period
randomized withdrawal trial proz.com
DRAFT ICH CONSENSUS P ICH Official web site
Evaluation of Dependence and Withdrawal in Clinical Trials and Human Dependence Study – Design and Considerations Alicja Lerner, a randomised study.
Bisphosphonates for Osteoporosis — Where Do We Go from
CDISC Guidelines for Annotating CRF
Long-term studies to show efficacy (and which are also needed for assessment of long term safety) would usually use active controls, preferably with a placebo-controlled randomized withdrawal study at the end of treatment to establish assay sensitivity (See ICH E10) and assess possible withdrawal effects. Another long-term active
Should the randomized withdrawal design for relapse
A 12-Week Randomized Controlled Trial With a 4-Week
In the study, extended-release naltrexone randomized-controlled study were related to induced or experienced withdrawal symptoms, which the study
FDA Approves Lofexidine Hydrochloride First Non-Opioid
DRAFT ICH CONSENSUS PRINCIPLE U S Food and Drug
FDA Guidance, Clinical Pharmacology, Regulatory Science Copy of a cover for an FDA Guidance for Industry Population PK + randomized withdrawal clinical trial
Ampio’s stock plunges below threshold after FDA
The FLEX and HORIZON-PFT trials used a randomized withdrawal design in which patients who had previously been receiving bisphosphonate treatment were enrolled in the extension periods and underwent repeated randomization to receive either placebo or continued bisphosphonate treatment.
FDA Approves Lofexidine Hydrochloride First Non-Opioid
Randomized Withdrawal Design and Randomized Blogger
Vyvanse FDA prescribing information side effects and uses
In this study, either Keppra or placebo was the prospective 8-week baseline period were randomized to either Keppra or to read the FDA-approved
Ampio’s stock plunges below threshold after FDA
Lofexidine Lessens Opioid Withdrawal Medpage Today
A double-blind placebo-controlled randomized withdrawal
to randomized control trial • Cons: – Limited experience – Ethical issues of placebo treatment • Depends on the 22 while maintaining comparable statistical power – Provides more information about efficacy – Provides information about need for continued treatment consequences of non-treatment – Same limitations as for Randomized Withdrawal
GUIDANCE DOCUMENT Withdrawal of Subjects from Research
A 12-Week Randomized Controlled Trial With a 4-Week
Lofexidine Lessens Opioid Withdrawal Medpage Today